Reaction Coordinate Diagrams and What They Say About Living Life
So this year, I took Organic Chemistry, and if there’s one fundamental concept or takeaway that I got from the class, it’s the importance of Reaction Coordinate Diagrams. For those who are unfamiliar, here’s a pic:
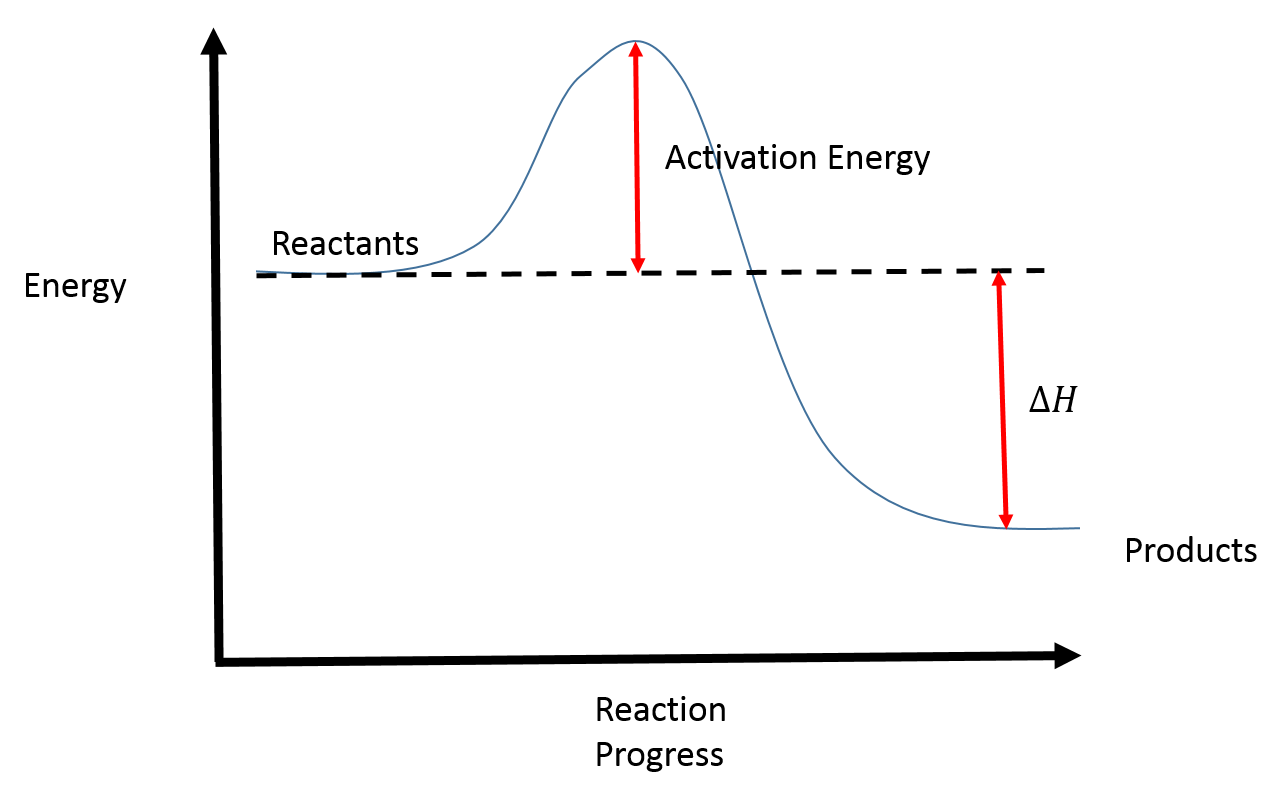
Basically, for any reactant to react, it has to have a certain activation energy. Usually, this activation energy comes in the form of heat or radiation. With enough energy, it overcomes the hump and usually goes to a lower energy state. That’s how catalysts work: by turning a single reaction into a multi-step reaction, lowering that activation energy hump so things move along faster.
Now that you have a basic understanding of this, now what does this have to do with life at all?
I am super lazy. I’m really good at lying in bed and moping around. Sometimes my mom yells at me saying if she weren’t around I would literally get nothing done. So clearly, the chemicals I play around with in the lab and I are similar: we’re trying to get to the lowest energy state.
So how do you get to the lowest energy state? Well easy, you need some activation energy. Maybe a catalyst to lower the activation energy. And what is a catalyst? Maybe it’s motivation, but let’s be real. Motivation comes and goes. And the key is what my mom told me. Without her, I’d get nothing done. She’s my catalyst! Now obviously, I can’t have my mom be my catalyst for everything. She does enough for me. So instead, I shape my environment. It’s so much easier to get something done if I’m already sitting at my desk. It’s so much easier to start an assignment once I open the google doc.
Catalyze your life. Twenty smaller humps is way easier than one big heap of activation energy. Oftentimes, the twenty humps will sum to more anyways. And at the end of the day, I get to trick myself into thinking that I’m saving energy. Lazy people for the win! Also, thank you Mr. Faulk for your insights in class. You are the 🐐.
